Arsenic contamination of water: Detection and treatment challenges
Arsenic contamination of water, whether surface water or ground water, affects many parts of France. Such contamination may be the result of anthropogenic causes, linked to mining operations for example, or of natural causes in relation to changes in geological formations, as is the case in many countries such as India, Pakistan and Chili. In partnership with the Nuclear Materials Authority in Egypt and Guangxi University in China, a team of researchers from IMT Mines Alès has developed processes for treating contaminated water and detecting arsenic.
Arsenic may be present naturally in water used for irrigation and animal-rearing. In the majority of cases, these concentrations are low enough – less than 0.01 milligrams per liter – that they do not pose a risk to humans. Hoever, overexposure can have dramatic effects on fauna, flora and human health, by causing cancer and dermatological conditions for example.
Emblematic cases of anthropogenic contamination have been reported in relation to mining activities. In 2019, near the gold mine in Salsigne, in the south of France, children were overexposed to arsenic, probably spread through dust and the dissolution of arsenic from mine tailings. But natural phenomena, such as the infiltration of rainwater, the erosion of geological formations and soil leaching may also be the cause of contamination in many countries like India, China and Chili.
“Unfortunately, the countries faced with this environmental and health challenge are often poor countries that don’t have access to the most advanced techniques for decontaminating water,” says Eric Guibal, a researcher at IMT Mines Alès. “In these countries, communities often rely on low-tech processes to reduce the toxicity of catchment water. Trade-offs are made between effectiveness and production and operation costs, for example through pumping and filtration using iron oxides” he explains. Along with a team of colleagues and in partnership with the Nuclear Materials Authority in Egypt and Guangxi University in China, he has contributed to the development of a number of innovative materials for the detection and treatment of arsenic in contaminated water.
Materials that “like” arsenic
“What we’re proposing is not a technique that can replace those we already have to capture arsenic, but a complementary technique in order to improve decontamination,” adds Eric Guibal. These research teams have developed a range of adsorbants, materials that can bind ions or molecules, in order to capture arsenic. Based on the use of biopolymers (extracts of seaweed and crustacean shells), they provide an alternative to those produced from petroleum resources. “Replacing petroleum-sourced materials with renewable resources is, in itself, an important challenge for the future,” he says.
Read more on I’MTech: When plants help us fight pollution
“Environmentally-friendly management has brought us to the limits of these materials; so as to avoid depleting the biotype, we limit the scope of our processes to applications such as polishing treatment and for “niches,” he explains. Combining these processes with more conventional techniques (including precipitation, filtration, etc.) makes it possible to significantly reduce the toxicity of effluents and their discharge and improve the quality of catchment water. The targeted field of application is therefore in line with local applications limited to areas where water quality is critical.
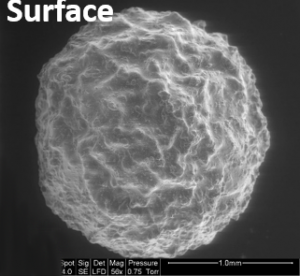
Various materials have been developed, for example combining the biopolymer - chitosan, a crustacean shell extract - with metal ions - such as molybdate - which have a particular affinity for arsenic ions. When they come together, the different ions form a complex, which, once immobilized, makes it possible to recover arsenic in solution. Furthermore, the synthesis of nanocomposites in the form of hollow spheres combining molybdate with other compounds (silicate and cellulose acetate) produces nano-objects that can be used for the detection, analysis and recovery of arsenic in solutions with low levels of contamination.
Another more recently developed material is based on the functionalization of a composite, a technique used to give a material certain specific properties. By combining seaweed with a synthetic polymer, this adsorbant material makes it possible to extract arsenic in order to decontaminate effluent or catchment water. The challenge is to release the arsenic once the material has been saturated, in order to concentrate it and recycle the adsorbant for new treatment cycles.
From the lab to the world
Another advantage of these technologies is that they offer a primary biological material that provides an alternative to the more polluting processes currently in use. But the innovation is struggling to gain acceptance outside the laboratory, in particular since manufacturers may be reluctant to change their processes. “It’s a process that companies don’t know about and there’s a fear that there won’t be the same reproducibility ,” he adds. The variability of the resource can be a deterrent for manufacturers in terms of ensuring production and reproducible properties.
But beyond that, there is also a certain competitiveness in terms of production lines. Manufacturers already have a process that works and for which there is a market, and may not necessarily feel a need to change in order to find alternatives to petroleum-based processes. “And yet, they must be proactive and anticipate the future of petroleum-based resources,” says Eric Guibal. “There are also profitability issues involved, and if the environmental cost of the processes were taken into account, this innovation may be more appealing,” he concludes.
Tiphaine Claveau for I’MTech





Leave a Reply
Want to join the discussion?Feel free to contribute!